
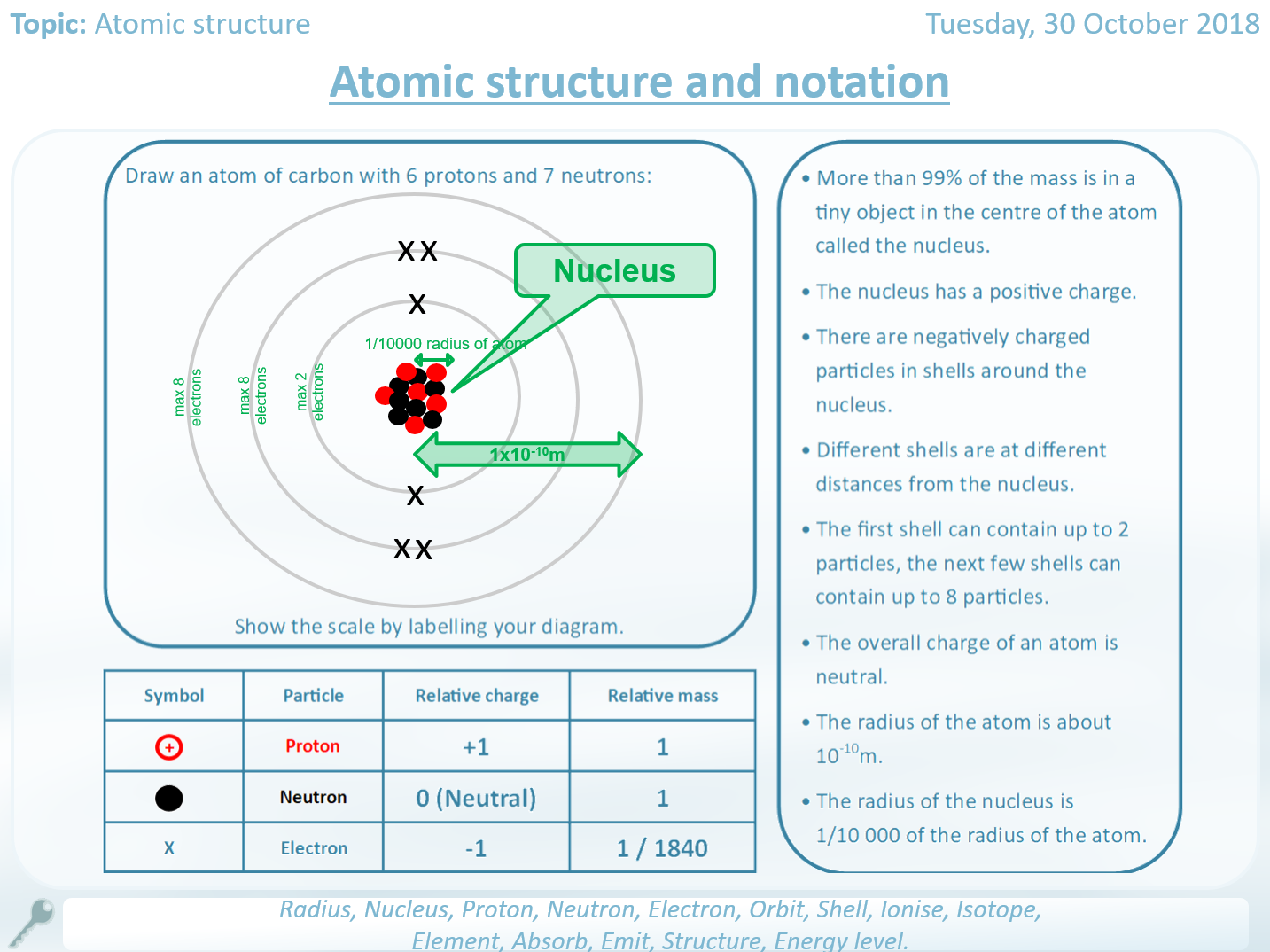
Since the nucleus contains protons and neutrons, most of the mass of an atom is concentrated in its nucleus. The mass of an electron is very small compared to a proton or a neutron. The relative mass of a proton is 1, and a particle with a relative mass smaller than 1 has less mass. Define the term relative atomic mass and why it takes into account the abundance of isotopes of the element. Describe isotopes as atoms of the same element with different numbers of neutrons. The number of protons in an atom is always the same as the. Around this nucleus is a number of electrons that differs depending on the element of the periodic table. An atom is a central positively charged nucleus that is made of protons and neutrons. Instead of writing their actual masses in kilograms, we often use their relative masses. Calculate the number of protons, neutrons and electrons in an atom when given its atomic number and mass number. What is atomic structure Atomic structure is the make-up of an atom and what it consists of. The masses of subatomic particles are very tiny. The structure of a carbon atom, not drawn to scale Search: Atomic Structure Multiple Choice Questions And Answers Choice Atomic Multiple Answers And Structure Questions .it Views: 10110 Published: Author: .it Search: table of content Part 1. The nuclei of most atoms also contain neutrons. The nuclei of all atoms contain subatomic particles called protons. the radius of a nucleus (1 × 10 -14 m) is less than \(\frac\) of the radius of an atomįor comparison, the radius of a typical bacterium is 1 × 10 -6 m and the radius of a human hair is about 1 × 10 -4 m.the radius of an atom is about 0.1 nm (1 × 10 -10 m).The nucleus is tiny compared to the atom as a whole: This is surrounded by electrons arranged in shells.


 0 kommentar(er)
0 kommentar(er)
